Aug 14, 18 · When polymerization is initiated (triggered) by peroxide, free radicals are formed as intermediates The number of unsaturated compounds such as alkene and alkadiene makes addition polymerization can be carried out in the presence of peroxides such as benzoyl peroxide or acetyl peroxide with high temperatures and pressuresUnderstand the ketoenol tautomerisation of acetylacetone and determine the electronic structure of their compound using Evans method and the Spinsolve NMR spectrometer Figure 1 Ketoenol equilibrium of acetylacetone and formation of acetylacetonate anion The complete addition of hydrogen peroxide should take around 30 minutes ContinueAnd cure times and it is mailnly used for polyester;
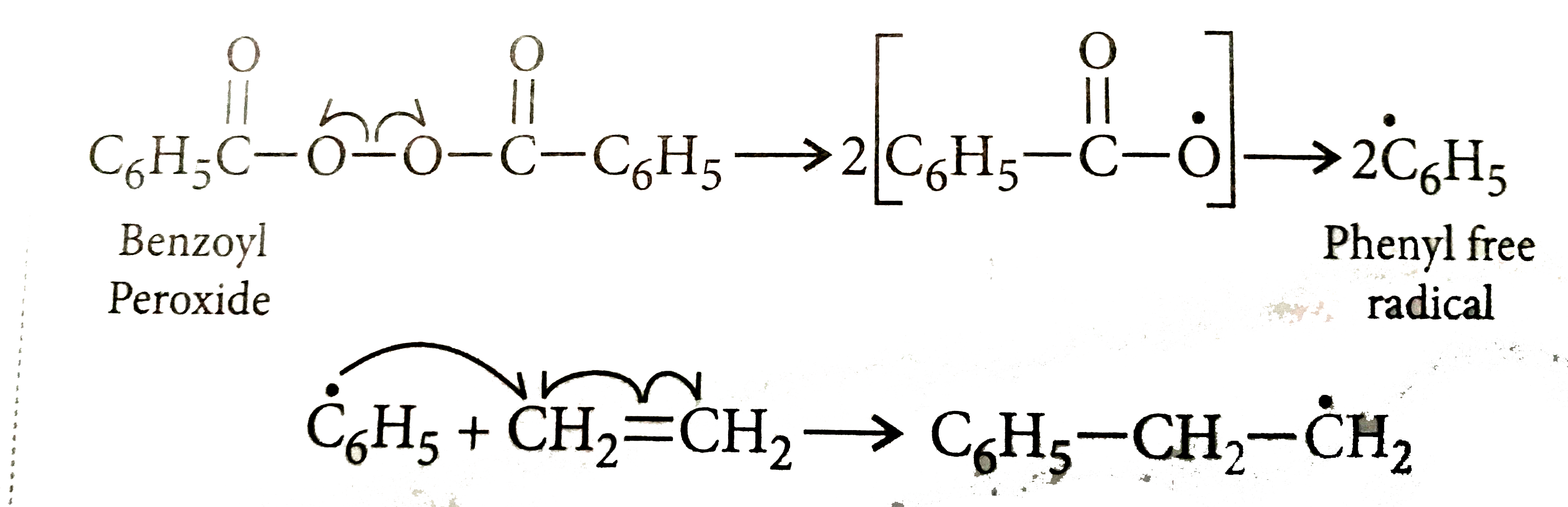
During Addition Polymerisation Of Ethene Molecules The Initiator
Acetyl peroxide structure
Acetyl peroxide structure-Used as an initiator and catalyst for resinsApr 10, 19 · Salicylic acid and benzoyl peroxide are two of the most wellknown acnefighting ingredients Widely available over the counter (OTC), they both help clear mild acne and prevent future breakouts



Peracetic Acid Peroxyacetic Acid Ch3co3h Cas No 79 21 0 Acetic Peroxide Acetyl Hydroperoxide In Main Pusa Road New Delhi Acuro Organics Limited Id
With organic substances pure hydrogen peroxide is a powerful and valuable oxidising agent;Visit ChemicalBook To find more ACETYL PEROXIDE() information like chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight, physical properties,toxicity information,customs codes You can also browse global suppliers,vendor,prices,Price,manufacturers of ACETYL PEROXIDE() At last,ACETYL PEROXIDEAcetylacetone Peroxide or Acetyl Acetone Peroxide Manufacturers, SDS GHS MSDS Sheet Muby Chemicals of Mubychem Group, established in 1976, is the original manufacturers of Specialty Chemicals, Pharmaceutical Excipient, Fragrance Food & Flavor chemicals, Reagent Grade Chemicals, Shale Gas Fracturing Chemicals in India Mubychem Group has several manufacturing
The fatty acidCoA molecule is degraded into acetyl CoA molecules by a recurring cyclic sequence of four reactions The end product of each cycle is the fatty acid shortened by 2 carbons and acetyl CoA The series of reactions is also known as the betaoxidation pathway because the major reaction site is the betacarbon or #3 carbon from thePeroxy acid, any of a class of chemical compounds in which the atomic group ―O―O―H replaces the ―O―H group of an oxy acid (a compound in which a hydrogen atom is attached to an oxygen atom by a covalent bond that is easily broken, producing an anion and aAcetyl benzoyl peroxide agent detailed information in HazMap database
Luperox® Curing of Unsaturated Polyester Resins Author Arkema IncAcetyl acetone peroxide (aap) cas no structure benzoyl peroxide (bpo) benzoyl peroxide (bpo) cas no structure click here to view Acetyl Acetone Peroxide ;Peroxide Compound containing the peroxy group (OO), chainlike structure, containing two oxygen atoms, each of which is bonded to the other and to a radical or some element It is considered that hydrogen peroxide is the starting material to prepare organic and inorganic peroxides commercially
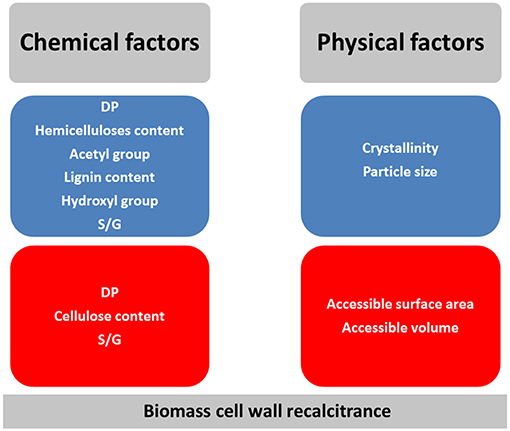


Frontiers Lignocellulosic Biomass Understanding Recalcitrance And Predicting Hydrolysis Chemistry


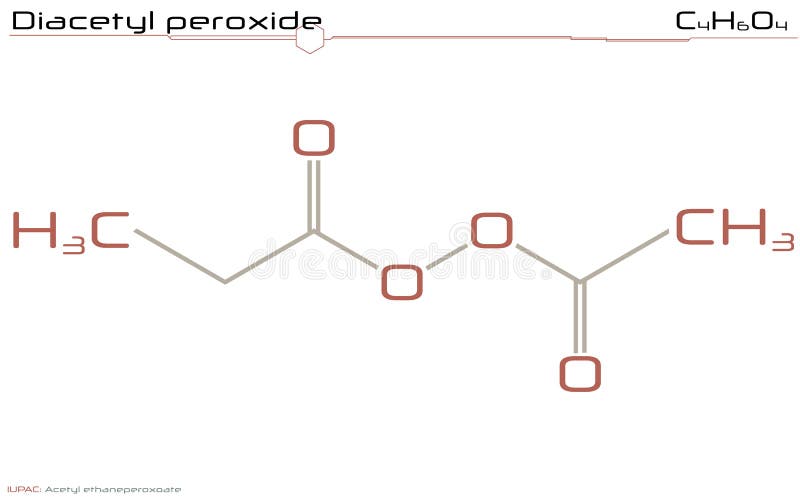
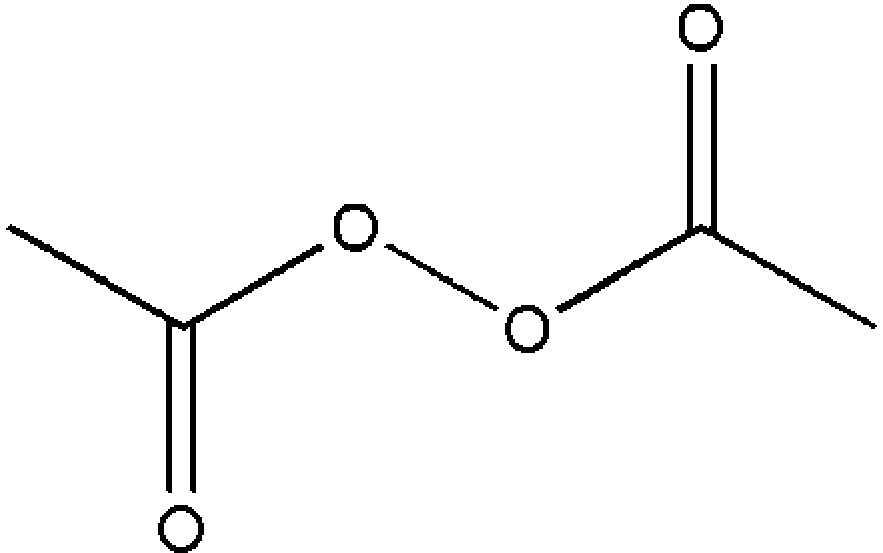
Diacetyl Peroxide
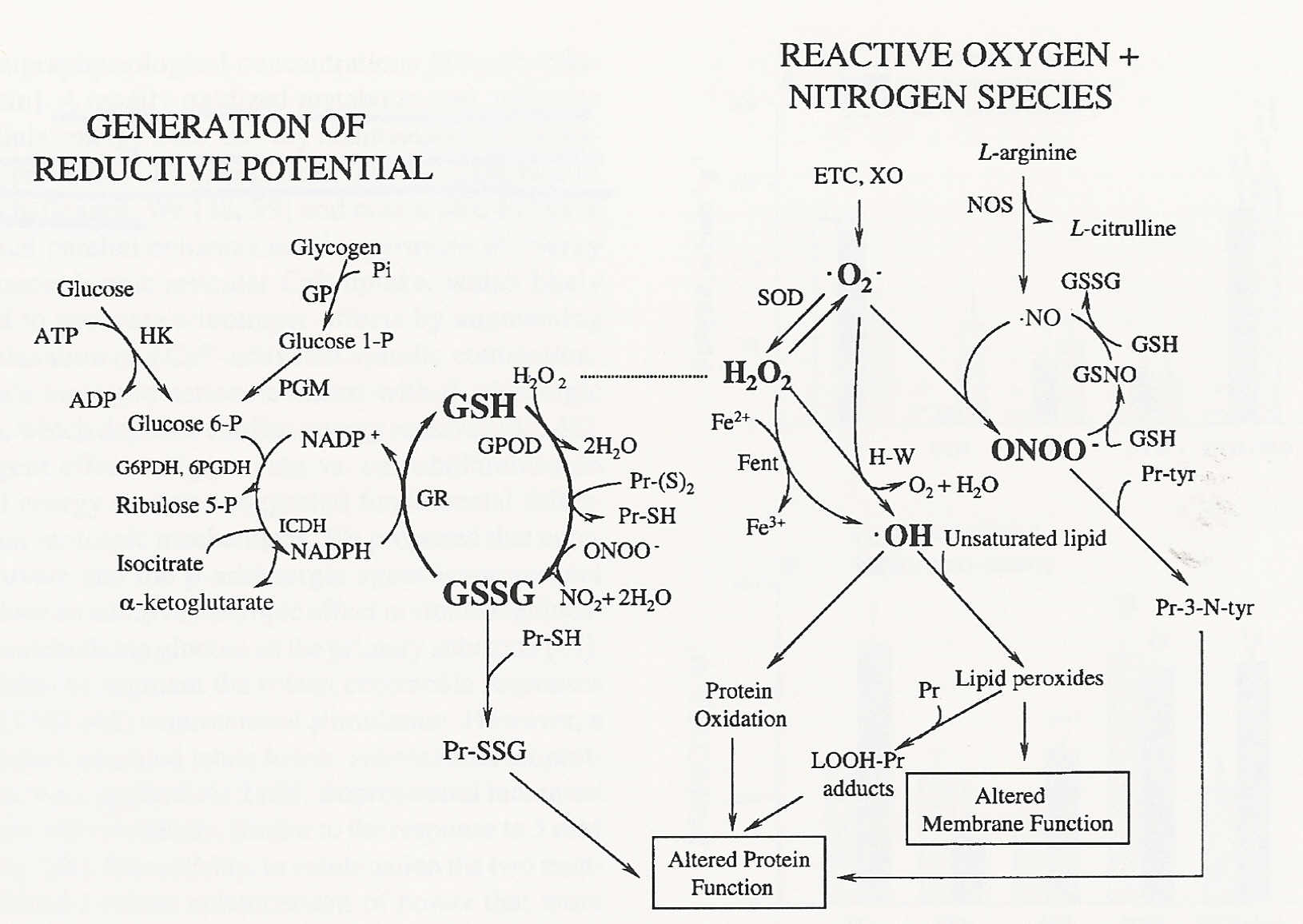
Acetanilide is a derivative of aniline, where one of the hydrogens on the nitrogen atom has been replaced with an acetyl group Acetanilide has a wide variety of uses and is a useful buildingAcetyl peroxide is used as a catalyst for resins Chemical Formula C 4 H 6 O 4 Other names Acetyl Peroxide Layman's explanation Acetyl peroxide is a colorless liquid with a pungent odor It is generally stored as a 25% solution in dimethyl phthalate to prevent detonation It may explode if heated, or in contact with combustible materialsMar 02, · Glutathione (γLGlutamylLcysteinylglycine) is a small amino acid containing molecule (peptide) comprised of one molecule of Lglutamic acid, Lcysteine, and Glycine eachThe molecule is found in the food supply and in the human body where it acts as an antioxidant The 'glutathione system' comprises the enzymes that synthesize glutathione within a cell as well as
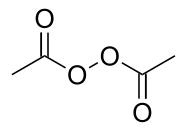


Diacetyl Peroxide Wikipedia



Organic Peroxide Wikiwand
321 Physical Description Acetyl benzoyl peroxide appears as a solution containing less than 40% by mass of the white crystalline solid in a nonvolatile solvent Dilution moderates reactivity of the pure solvent Irritating to the skin, eyes and mucous membranesAnother general method for the preparation of such organic peracids is by the interaction of hydrogen peroxide withAcetyl Acetone Peroxide Ask Price Samuh Laxmi Chemicals Bom P Ltd Abhaychand Gandhi Marg, Masjid Bunder, Mumbai 401, Sujata Chamber, 4th Floor, 1/3,, Abhaychand Gandhi Marg, Masjid Bunder, Mumbai , Dist Mumbai, Maharashtra
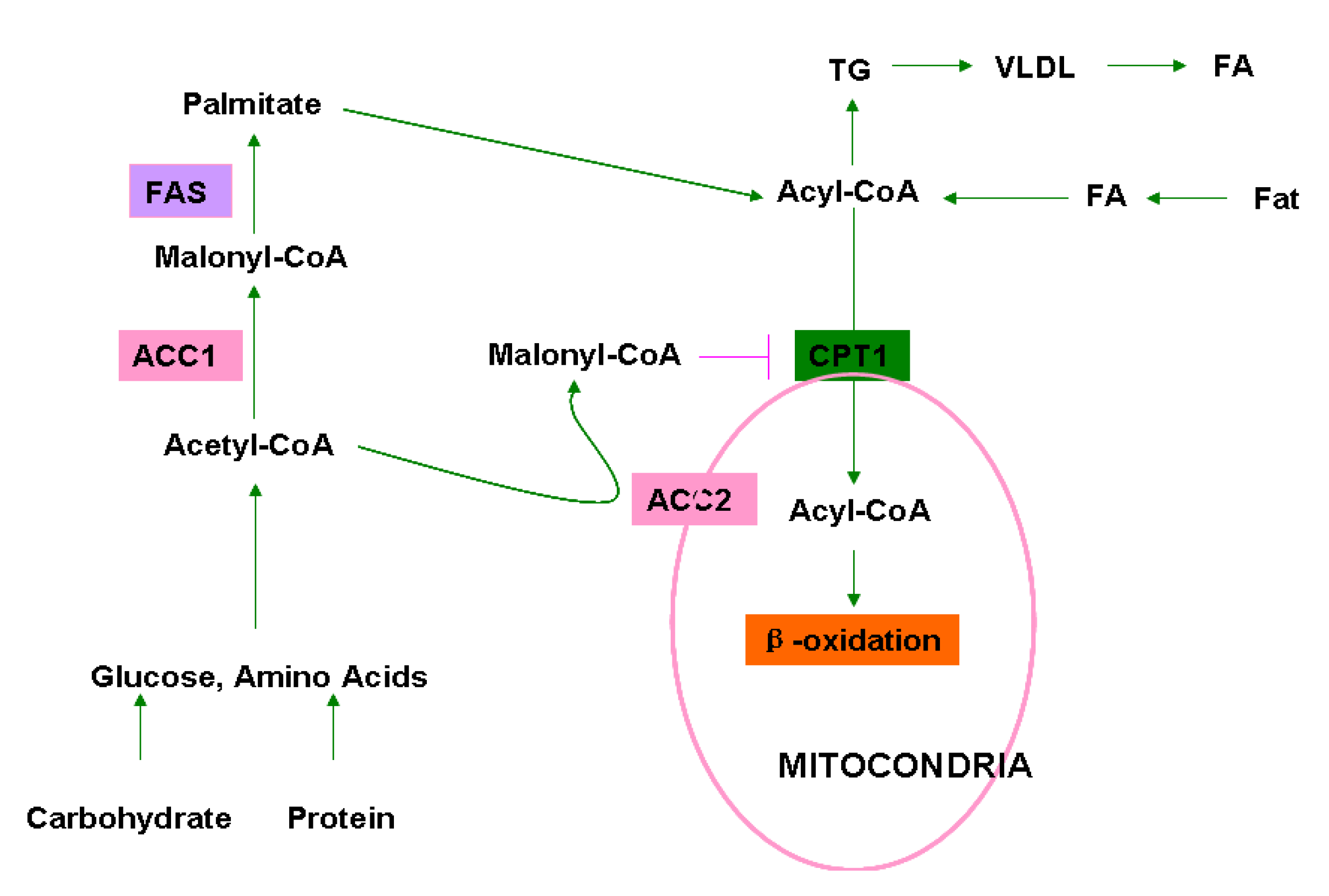


Molecules Free Full Text Chemical Genetics Of Acetyl Coa Carboxylases Html



Acetyl Benzoyl Peroxide Structure C9h8o4 Over 100 Million Chemical Compounds Mol Instincts
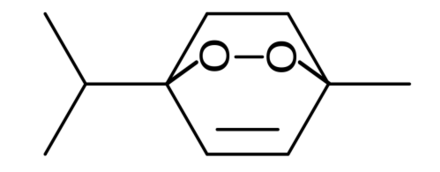
Acetyl peroxide, the lowest member of the primary diacyl peroxide series, was found to decompose only homolytically The decomposition of δphenylvaleryl peroxide, a higher primary homologue, was mainly by homolytic cleavage of the O O bond, however 30% of the ester formed was a product of heterolysis involving carboxy inversionSorption to aerosols (25 Dec C)AEROWIN v100 Vapor pressure (liquid/subcooled) 524E003 Pa (393 mm Hg) Log Koa (Koawin est ) 2953 Kp (particle/gas partition coefStructure, properties, spectra, suppliers and links for Acetone peroxide, Triacetone triperoxide,


Acetozone C9h8o4 Chemspider



I1qxs2ue2vye8m
Earliest use found in Henry Roscoe (13–1915), chemist and university administratorAcetal (Polyoxymethylene) Chemical Compatibility Chart Check the chemical compatibility of Acetal and Delrin® with various chemicals, solvents, alcohols and other products Shop Acetal Please Note The information in this chart has been supplied by reputable sources and is to be used ONLY as a guide in selecting equipment for appropriate chemical compatibilityAcetyl Acetone Peroxide is Liquid Solution in alcohol or Dimethyl phthalate It is a Curing agent for unsaturated polyester Peroxides, such as Acetyl Acetone Peroxide, are good oxidizing agentsOrganic compounds can ignite on contact with concentrated peroxides



Chemical Structure Of Melatonin N Acetyl 5 Methoxy Tryptamine Download Scientific Diagram
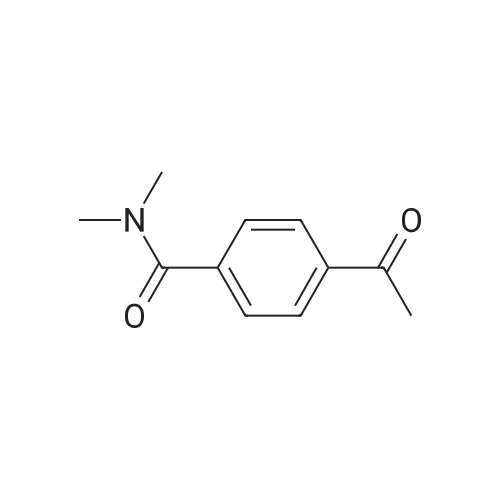


09 2 4 Acetyl N N Dimethylbenzamide Ambeed
The CLP Regulation ensures that the hazards presented by chemicals are clearly communicated to workers and consumers in the European Union through classification and labelling of chemicalsShare with your friends Share 0ACETYL BENZOYL PEROXIDE SOLUTION
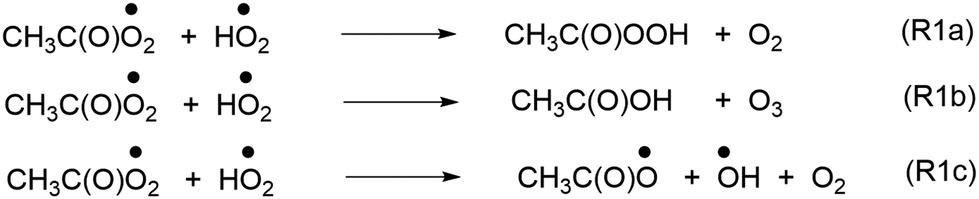


Unimolecular Decomposition Of Acetyl Peroxy Radical A Potential Source Of Tropospheric Ketene Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 D0cpj
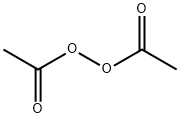


Acetyl Peroxide 110 22 5
A kinetic study of the decomposition of benzoyl peroxide assisted by Lewis acids Polymer Bulletin 1980, 33 (12) , 2735 DOI /BF James Weaver, James Meagher, Robert Shortridge, Julian Heicklen The oxidation of acetyl radicalsNacetyl glucosamine is a chemical that comes from the outer shells of shellfish It can also be made in labs Don't confuse Nacetyl glucosamine with other forms of glucosamine, such asPeroxides, such as ACETYL ACETONE PEROXIDE, are good oxidizing agents Organic compounds can ignite on contact with concentrated peroxides Strongly reduced material such as sulfides, nitrides, and hydrides may react explosively with peroxides There are few chemical classes that do not at least produce heat when mixed with peroxides
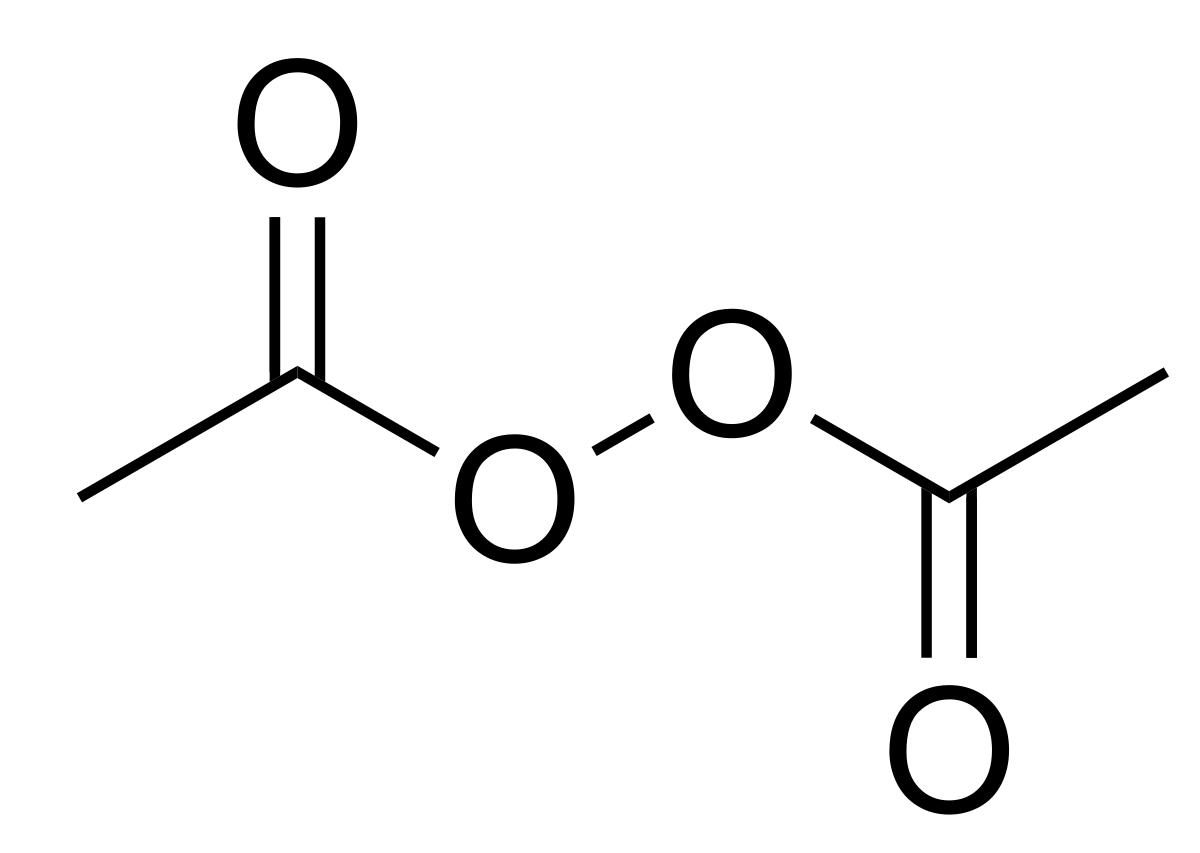


Diacetyl Peroxide Wikipedia
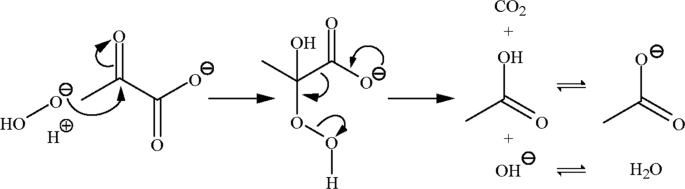


Reaction Rate Of Pyruvate And Hydrogen Peroxide Assessing Antioxidant Capacity Of Pyruvate Under Biological Conditions Scientific Reports
Looking for acetyl peroxide?Changes will be taking place on SigmaAldrichcom on June 5, 21 that include visual and functional updates All registered users will be prompted to reset their password the first time logging in to the new siteAcetyl peroxide definition is a lowmelting crystalline compound (CH3CO)2O2 used especially for initiating vinyltype polymerizations
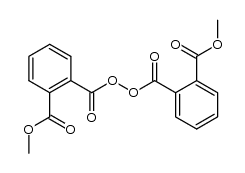


Acetyl Benzoyl Peroxide Cas 644 31 5 Chemsrc



Chemical Structure Of Hyaluronan A D Glucuronic Acid B Download Scientific Diagram
Nature acetyl peroxide also known as methyl benzene The temperature ranged from 36 to 37 ° C Boiling Point (2533Pa) 130 ° C In the case of water or warmth Department decomposition Dissolved carbon tetrachloride, chloroform, ethyl ether, oilsPeroxide, acetyl cyclohexylsulfonyl Acetyl cyclohexanesulphonyl peroxide Acetyl cyclohexanesulfonyl peroxide Lupersol 228ZNOW Foods NAcetyl Cysteine NAcetyl Cysteine (NAC) is a stable form of the non essential amino acid LCysteine A sulfurcontaining amino acid that acts as a stabilizer for the formation of protein structures, also necessary for the formation of Glutathione
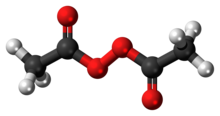


Diacetyl Peroxide Wikipedia



Structure Of Tertbutyl Peroxide And Acetyl Peroxide Chemistry Polymers Meritnation Com
Recommendation for diacetyl peroxide usage levels up to not for fragrance use Recommendation for diacetyl peroxide flavor usage levels up to acetyl ethaneperoxoate acetyl peroxide acetylperoxide diacetylperoxide dimethylperoxyanhydride ethanoyl peroxide peroxide, diacetyl peroyl A Articles None found yet NotesIt will oxidise acetyl chloride to peracetic acid, CH 3 C O 3 H , and acetyl peroxide ( C H 3 C O ) 2 O 2 , volatile unstable liquids;A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together The ACETYL BENZOYL PEROXIDE molecule contains a total of 21 bond (s) There are 13 nonH bond (s), 8 multiple bond (s), 4 rotatable bond (s), 2 double bond (s), 6 aromatic bond (s) and 1 sixmembered ring (s)



Solved Write A Structure And Name Of The Major Product Ex Chegg Com



The Chemistry Of Peresters Locklear European Journal Of Organic Chemistry Wiley Online Library
Nov 25, 19 · Tooth hypersensitivity and pain are undesirable side effects of bleaching agents in humans The aim of this study is to implement strategies to counter such side effects, and to demonstrate the efficacy and mechanisms of action of Nacetyl cysteine (NAC) in countering the side effects of clinically used bleaching agents In a series of in vitro experiments, animal modelAcetyl benzoyl peroxide definition is a white crystalline compound CH3COO2COC6H5 that is explosive when pure and is used in germicidal preparations and forSummary AcetylCoA carboxylase (ACC) is a complex multifunctional enzyme system ACC is a biotincontaining enzyme which catalyzes the carboxylation of acetylCoA to malonylCoA, the ratelimiting step in fatty acid synthesis There are two ACC forms, alpha and beta, encoded by two different genes ACCalpha is highly enriched in lipogenic


Organic Peroxide Stock Illustrations 65 Organic Peroxide Stock Illustrations Vectors Clipart Dreamstime
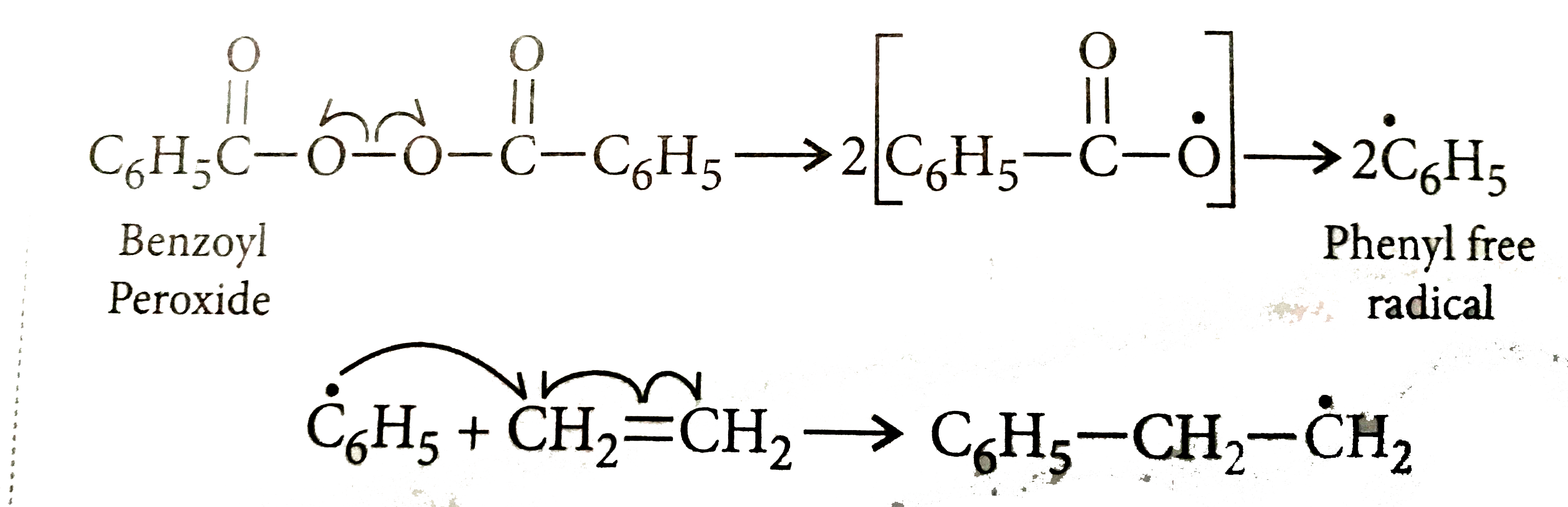


During Addition Polymerisation Of Ethene Molecules The Initiator
The overall structure of HRP is vital to the function of the system, but the heme active site and the conserved amino acid residues in the heme environment are the standard for peroxidase chemistry HRP enzymes are large, highly glycosylated hemecontaining enzymes (~44 kDa) and contain one PPIX heme prosthetic groupMar 11, 15 · structure of tertbutyl peroxide and acetyl peroxide?The pure material of Acetyl peroxide (CAS NO) should not be handled by untrained persons It's unstable as the pure material and incompatible with organic materials Reactivity Profile Peroxides, such as Acetyl peroxide (CAS NO), are good oxidizing agents Organic compounds can ignite on contact with concentrated peroxides



Peroxy Group An Overview Sciencedirect Topics
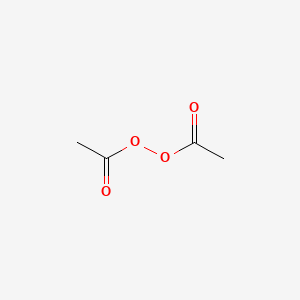


Diacetyl Peroxide C4h6o4 Pubchem
Find out information about acetyl peroxide 2O2 Colorless crystals with a melting point of 30°C;Earliest use found in Henry Roscoe (13–1915), chemist and university administrator* Acetyl Benzoyl Perxoide must be stored to avoid contact with IGNITION SOURCES, MOISTURE, WATER or STEAM since violent reactions occur * Acetyl Benzoyl Perxoide is not compatible with STRONG BASES (such as SODIUM HYDROXIDE and POTASSIUM HYDROXIDE) * Acetyl Benzoyl Perxoide is an Organic Peroxide which can detonate if shocked or heated



Structures Of Tyrosine N Acetyl Tyrosine Gly Tyr Glu Tyr Tyr Arg Download Scientific Diagram
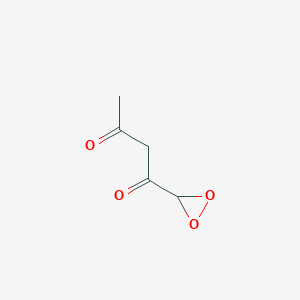


Acetylacetone Peroxide C5h6o4 Pubchem
Soluble in alcohol and ether;Acetyl peroxide acetylperoxide Diacetyl peroxide Wiki NO Hydrocarbon Biodegradation (BioHCwin v101) Structure incompatible with current estimation method!Chemistry Chemistry Principles and Reactions An objectionable component of smog is acetyl peroxide, which has the following skeleton structure (a) Draw the Lewis structure of this compound (b) Write the bond angles indicated by the numbered angles
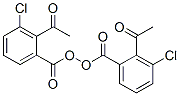


Acetyl M Chlorobenzoyl Peroxide Cas 777 05 9



Diacetyl Peroxide


Peroxide Stock Illustrations 300 Peroxide Stock Illustrations Vectors Clipart Dreamstime
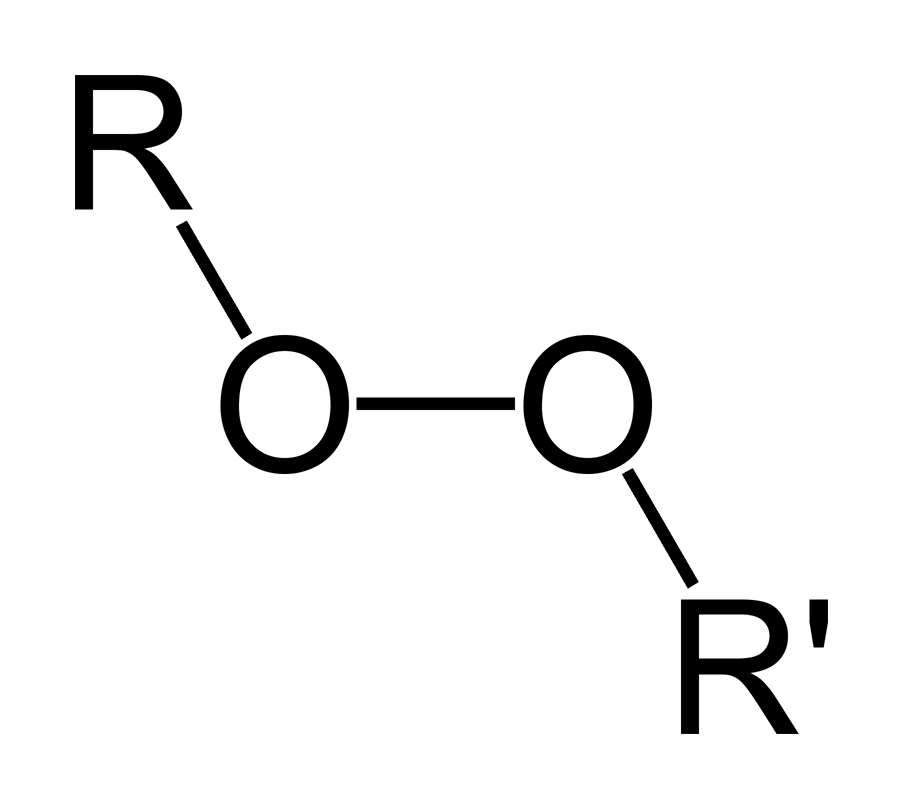


Organic Peroxide Wikipedia



Acetyl Structure



Scielo Brasil Photo Chemistry Without Light Photo Chemistry Without Light


Organic Peroxide Wikiwand
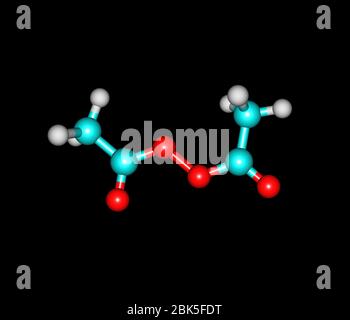


Diacetyl Peroxide Is An Organic Peroxide That Is A Crystalline Sand Like Solid With A Sharp Odor Stock Photo Alamy



Peracetic Acid Peroxyacetic Acid Ch3co3h Cas No 79 21 0 Acetic Peroxide Acetyl Hydroperoxide In Main Pusa Road New Delhi Acuro Organics Limited Id



Structure Of Melatonin N Acetyl 5 Methoxytryptamine Download Scientific Diagram


N Acetylcysteine Nac
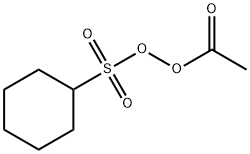


Acetyl Cyclohexane Sulfonyl Peroxide 3179 56 4
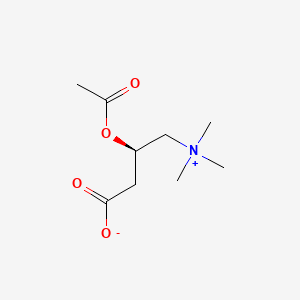


Acetyl L Carnitine C9h17no4 Pubchem


2 2 52 1 17 Adr 17 English
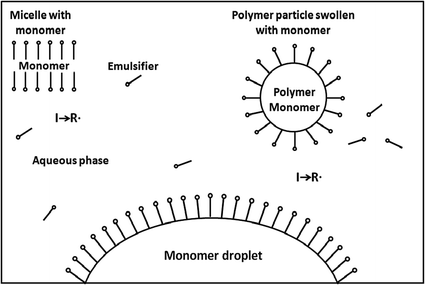


Radical Chain Polymerization Springerlink



Diacetyl Peroxide C4h6o4 Pubchem
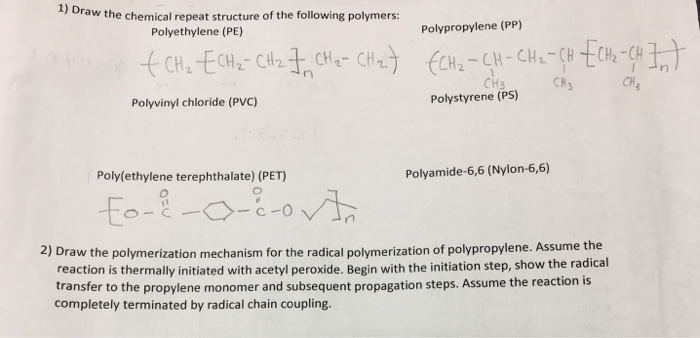


Solved 1 Draw The Chemical Repeat Structure Of The Follo Chegg Com



Solved 17 Structure On The Side Represents A Aconitase Chegg Com


Organic Syntheses Procedure
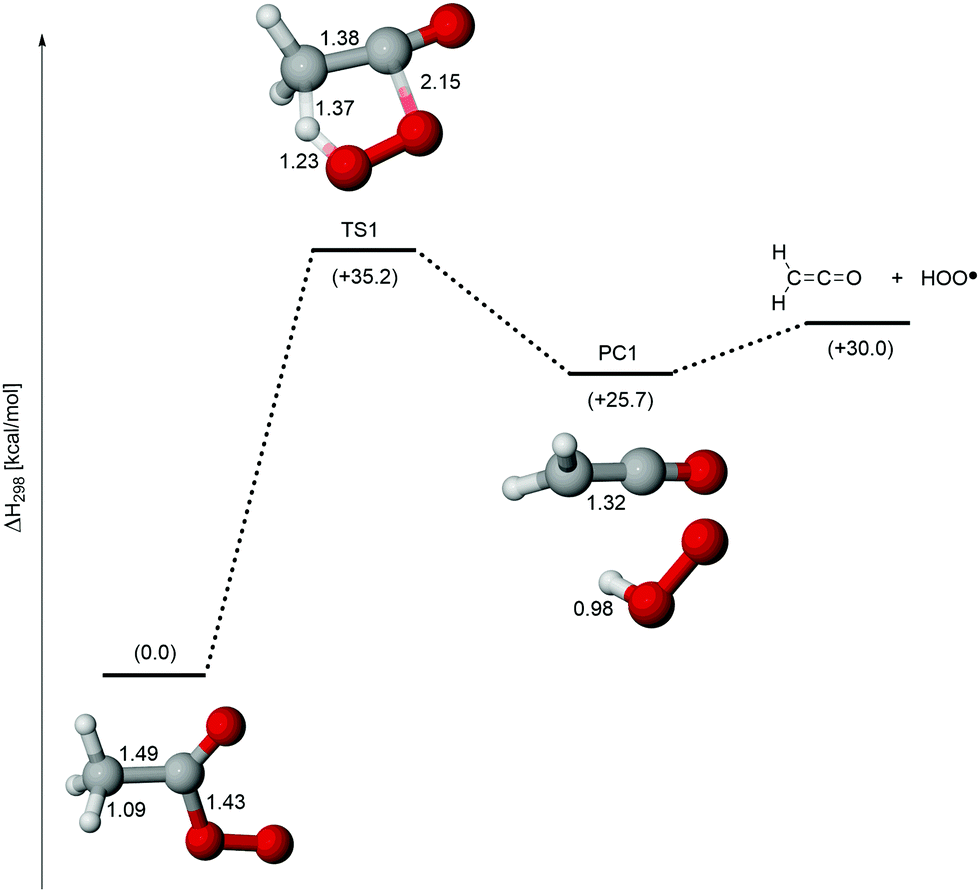


Unimolecular Decomposition Of Acetyl Peroxy Radical A Potential Source Of Tropospheric Ketene Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 D0cpj
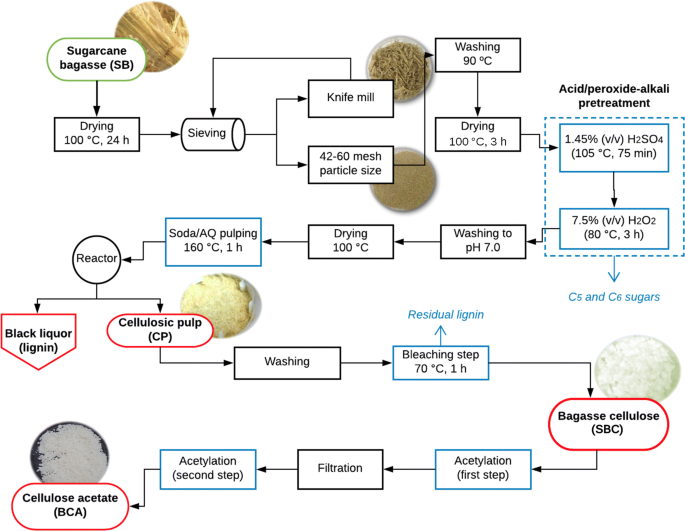


Investigating Acid Peroxide Alkali Pretreatment Of Sugarcane Bagasse To Isolate High Accessibility Cellulose Applied In Acetylation Reactions Springerlink



Remarkablz Com Henry Cecil Mcbay
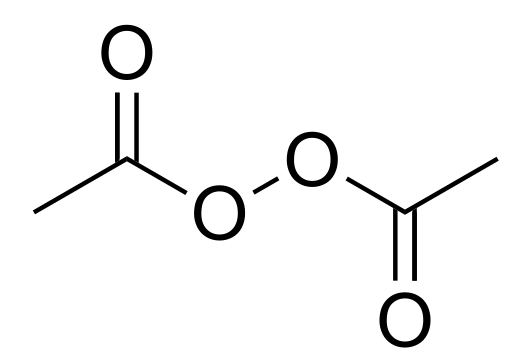


File Diacetyl Peroxide Svg Wikipedia
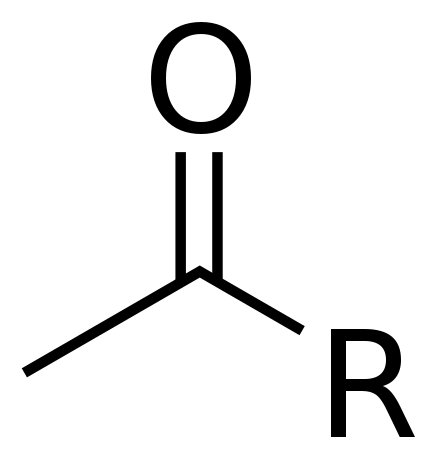


Acetyl Wikiwand



Acetyl Value And Peroxide Value Defination Principle Material Procedure Calculation Signification Youtube



The Chemistry Of Peresters Locklear European Journal Of Organic Chemistry Wiley Online Library



Acetyl Benzoyl Peroxide Cas 644 31 5 Chemsrc



Identification Of Macrodomain Proteins As Novel O Acetyl Adp Ribose Deacetylases Journal Of Biological Chemistry



Chemical Structure Of Glucosamine A And N Acetyl Glucosamine B Download Scientific Diagram


Organic Syntheses Procedure
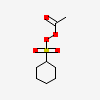


Peroxide Acetyl Cyclohexylsulfonyl C8h14o5s Pubchem



The Chemistry And Biological Activities Of N Acetylcysteine Sciencedirect



Organosulfur Compound Definition Structures Facts Britannica


Acetyl Peroxide Springerlink



Acetyl Chloride How Do You Make Acetyl Chloride Uses
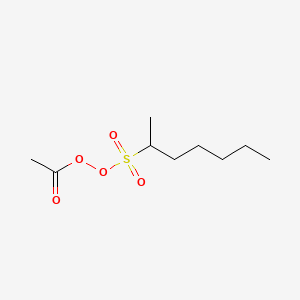


Acetyl Sec Heptylsulphonyl Peroxide C9h18o5s Pubchem



N Acetylcysteine In The Prevention Of Contrast Induced Nephropathy American Society Of Nephrology
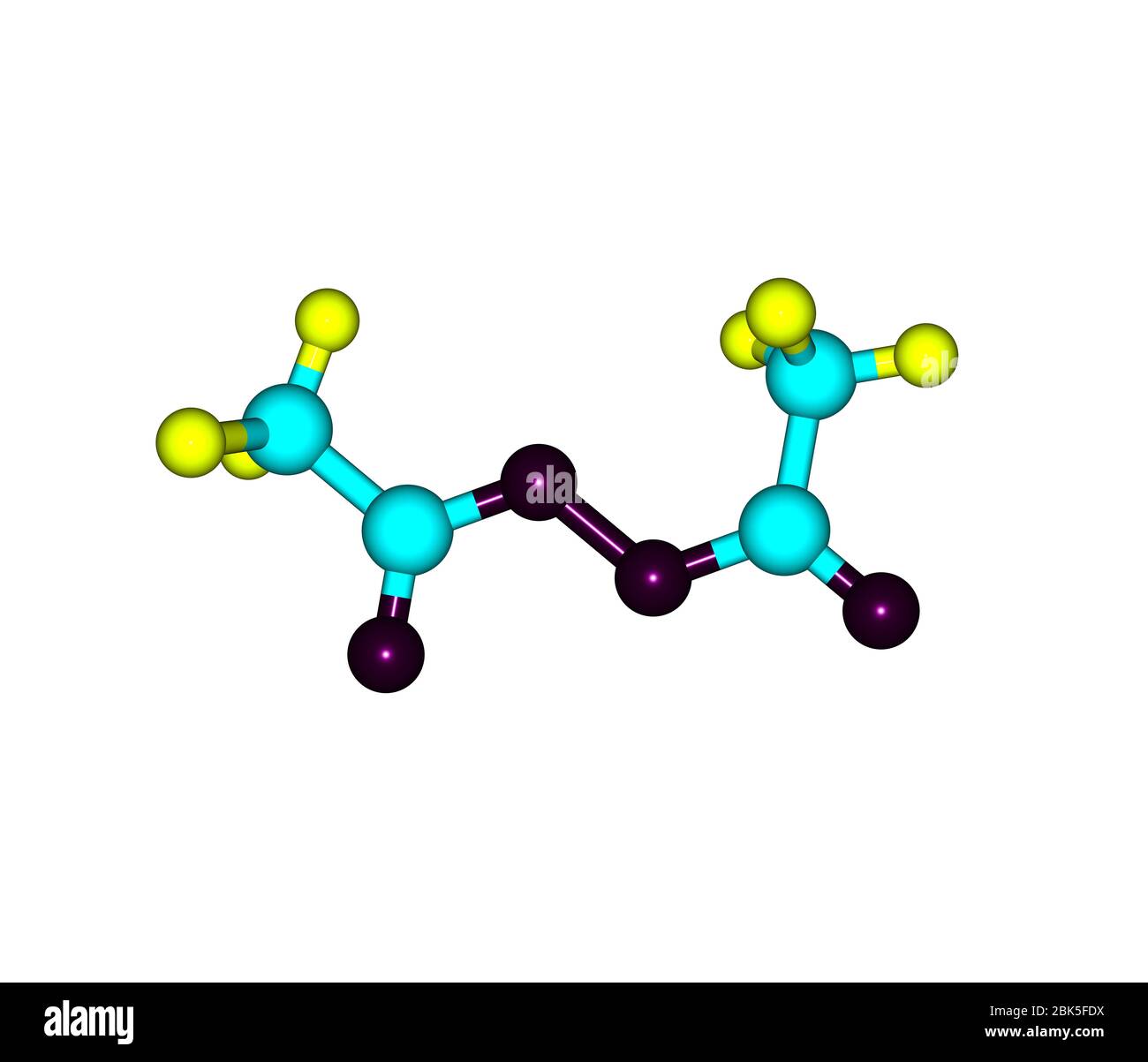


Diacetyl Peroxide Is An Organic Peroxide That Is A Crystalline Sand Like Solid With A Sharp Odor Stock Photo Alamy



Acetyl Bromide Cas 506 96 7 Chemsrc
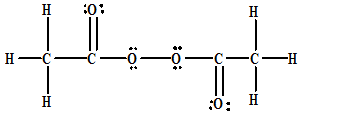


An Objectionable Component Of Smog Is Acetyl Peroxide Which Has The Following Skeleton Structure A Draw The Lewis Structure Of This Compound B Write The Bond Angles Indicated By The Numbered Angles
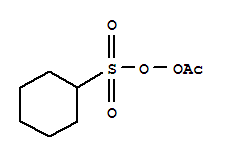


Cas No 3179 56 4 Peroxide Acetylcyclohexylsulfonyl Suppliers
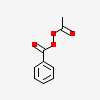


Acetyl Benzoyl Peroxide C9h8o4 Pubchem
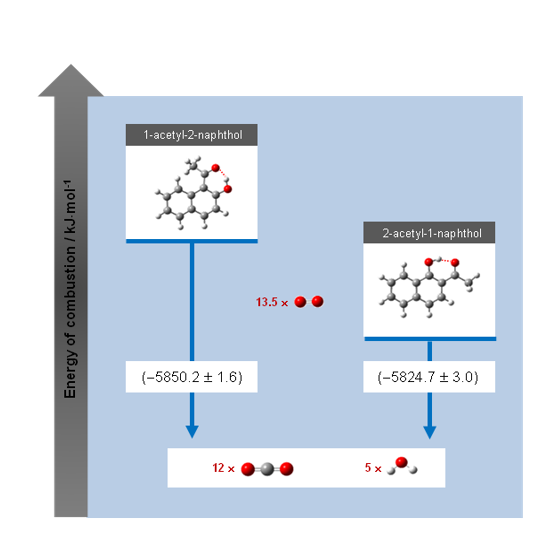


Molecules Free Full Text Structural And Energetic Insights On Two Dye Compounds 1 Acetyl 2 Naphthol And 2 Acetyl 1 Naphthol



The Structure Of Xylan And Mechanisms For Its Breakdown A Schematic Download Scientific Diagram


Concentration Dependent Dual Effects Of Hydrogen Peroxide On Insulin Signal Transduction In H4iiec Hepatocytes
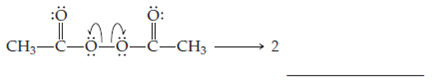


Solved The Homolysis Of The O O Bond In Diacetyl Peroxide Gives T Chegg Com



Acetone Molecule Acetic Acid Ball And Stick Model Acetyl Hexapeptide3 Chemistry Acid Acetic Acid Png Pngwing
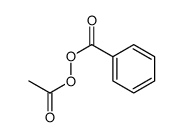


Acetyl Benzoyl Peroxide Cas 644 31 5 Chemsrc



Calcium Peroxide An Overview Sciencedirect Topics
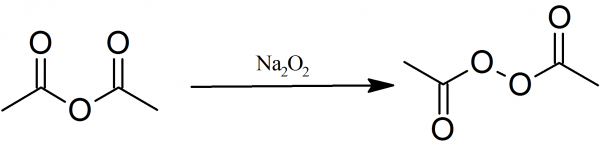


Preparation Of Acetyl Peroxide



644 31 5 Acetyl Benzoyl Peroxide C9h8o4 밀도 Nmr 분자 구조 분자식 비등점 인화점 녹는점 사전 Guidechem Com



Epa2 Reaction Of T Alkylhydrazinium Salts And Organic Peroxides To Foam Unsaturated Polyester Resins Google Patents
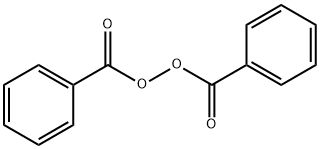


Benzoyl Peroxide Cas 94 36 0
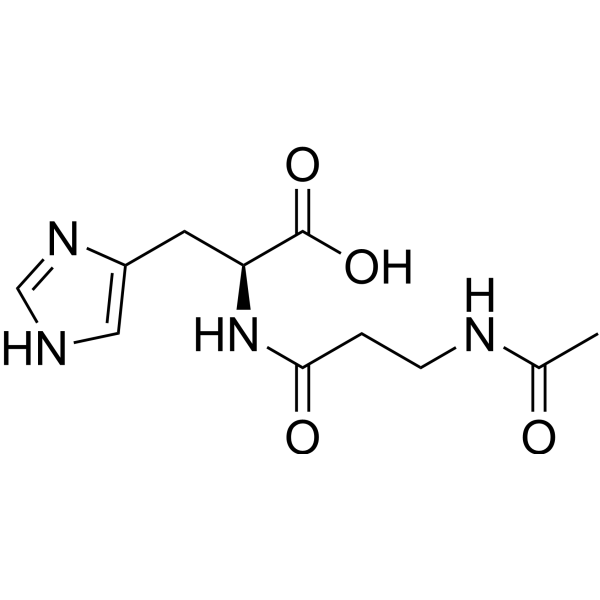


N Acetylcarnosine N Acetyl L Carnosine Peptide Medchemexpress



Acetyl Structure
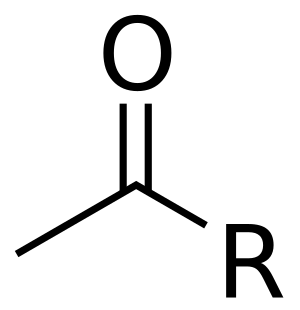


Acetyl Facts For Kids
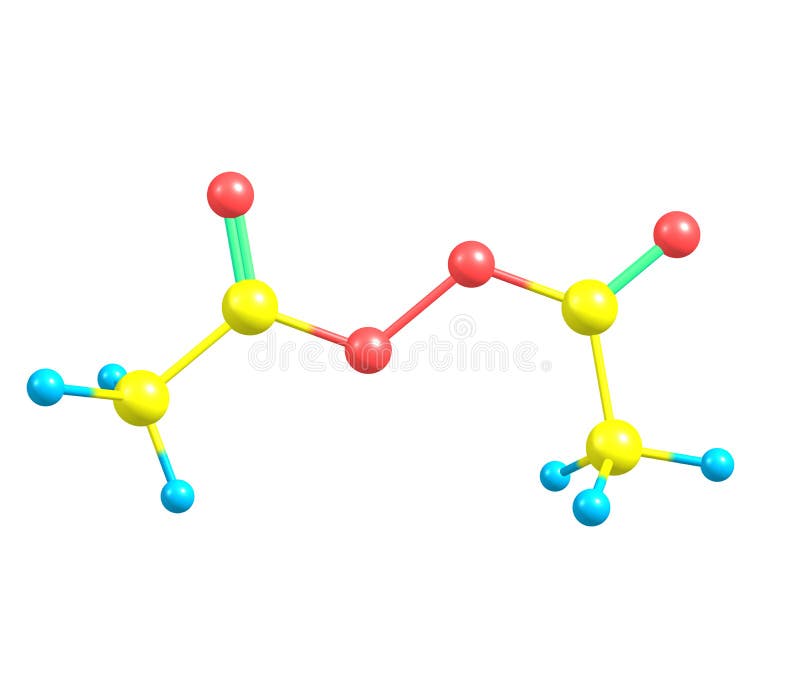


Peroxide Stock Illustrations 300 Peroxide Stock Illustrations Vectors Clipart Dreamstime


Diacetyl Peroxide C4h6o4 Chemspider



Acetyl Acetone Peroxide Properties Molecular Formula Applications Worldofchemicals



Acetyl Benzoyl Peroxide Structure C9h8o4 Over 100 Million Chemical Compounds Mol Instincts
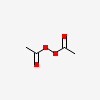


Diacetyl Peroxide C4h6o4 Pubchem
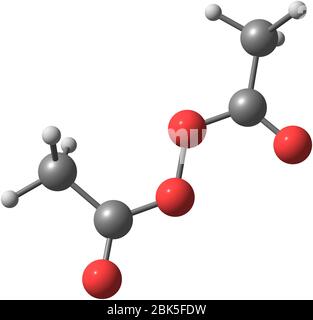


Diacetyl Peroxide Is An Organic Peroxide That Is A Crystalline Sand Like Solid With A Sharp Odor Stock Photo Alamy


Acetyl Benzoyl Peroxide Cas 644 31 5 Chemsrc
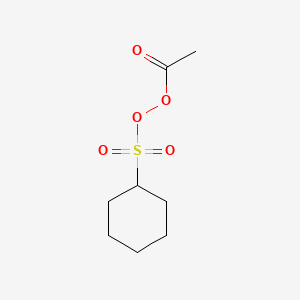


Peroxide Acetyl Cyclohexylsulfonyl C8h14o5s Pubchem
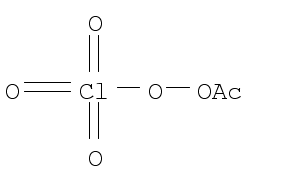


Peroxyacetyl Perchlorate Supplier Casno 43 9


Benzoyl Peroxide 94 36 0
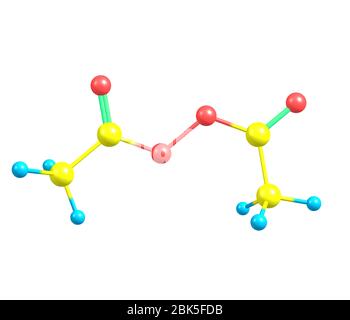


Diacetyl Peroxide Is An Organic Peroxide That Is A Crystalline Sand Like Solid With A Sharp Odor Stock Photo Alamy


Organic Syntheses Procedure


Chemidplus 110 22 5 Zqmigqncomnodd Uhfffaoysa N Diacetyl Peroxide Similar Structures Search Synonyms Formulas Resource Links And Other Chemical Information
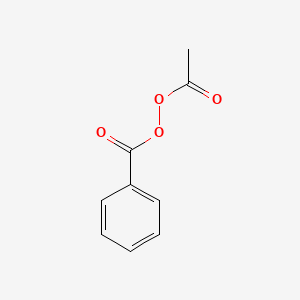


Acetyl Benzoyl Peroxide C9h8o4 Pubchem



Tetraacetylethylenediamine Wikipedia



0 件のコメント:
コメントを投稿